Abstract

Background: While remission rates for newly diagnosed AML have improved significantly with modern therapies, relapse remains a major cause of treatment failure and death. Post remission maintenance therapy in AML seeks to delay/prevent relapse with the goal of improving survival. From a historical cohort, among patients who did not receive allogeneic stem cell transplantation (aHSCT) in first remission (CR1), the median relapse-free survival (RFS) was 10m, 8m, and 5.7m for those who had received intensive therapy, lower-intensity therapy, or those with detectable minimal residual disease (MRD), respectively. Oral 5-azacytidine (CC-486) was shown to be effective in prolonging relapse-free and overall survival in AML patients in CR1 following intensive chemotherapy (QUAZAR AML-001). We designed this phase II trial to evaluate the efficacy and feasibility of low-dose IV/SQ 5-azacytidine (AZA) in combination with venetoclax (VEN) as maintenance therapy in AML patients who have achieved CR after high or low-intensity therapy.
Methods: Patients ≥ 18 years with AML were eligible for enrollment if they had achieved CR1 (CR/CRi), had ≥ 2 cycles of therapy prior to maintenance, and were not immediately eligible for aHSCT. Patients with detectable MRD in CR1 or beyond were also eligible. Cohort 1 included patients treated with intensive chemotherapy (defined as including standard or higher-dose cytarabine). Cohort 2 consisted of patients treated with low-intensity chemotherapy (defined as including a low-dose cytarabine (LDAC) or hypomethylating agent (HMA) backbone) who had received at least 2 cycles from time of CR/CRi to enrollment. Maintenance consisted of AZA 50 mg/m 2 IV/SQ on days 1-5 and VEN 400 mg PO on days 1-14 every 28 days, for up to 24 cycles. Patients who became eligible for aHSCT were taken off study and censored at the time of transplantation. VEN dosing was adjusted for patients on concomitant CYP3A inhibitors or those with severe cytopenias. The primary outcome was RFS. Secondary outcomes included overall survival (OS), event-free survival (EFS), and toxicity/safety. Flow cytometry-based minimal residual disease (MRD) and a next-generation sequencing (NGS)-based panel of 81 myeloid genes were performed on all patients.
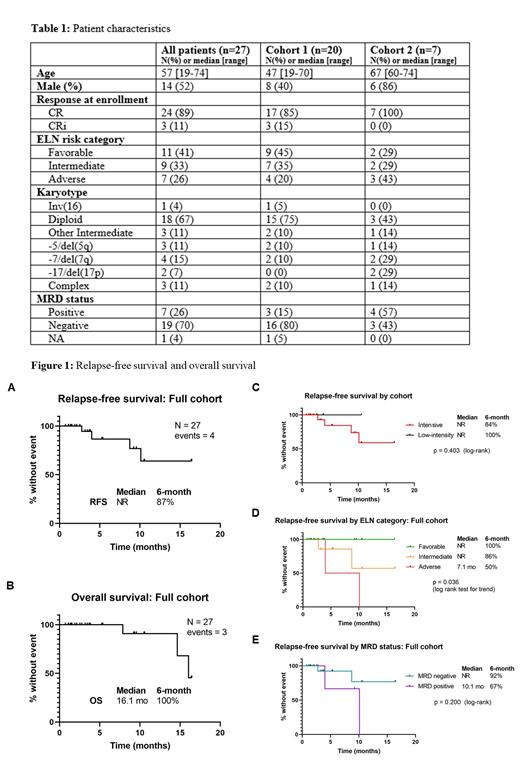
Results: 27 patients have been enrolled: 20 in cohort 1, 7 in cohort 2. Patient characteristics are presented in Table 1. 24 patients (89%) were in CR1 and 3 (11%) were in CR2. Prior to initiation of maintenance, patients had received a median of 1 (range 1-3) prior lines of therapy and 18 (67%) had been previously exposed to VEN. Patients have received a median of 3 (range 1-17 cycles) cycles of AZA-VEN maintenance. Starting cycle 1, 16 patients (59%) received 7 days and 11 patients (41%) received 14 days of VEN on protocol. 4 patients (15%) came off study to receive aHSCT. RFS and OS at 6m were 87% and 100% respectively for the full cohort. With a median follow-up of 3.8m (range 0.7-16.5m), the median OS was 16.1m and median RFS has not yet been reached (Figure 1, A-B); median RFS for patients with or without prior VEN was not reached and 10.1m, respectively (p = 0.3). RFS stratified by cohort, ELN risk category, and MRD status at enrollment is shown in Figure 1, C-E. 4 patients (15%) relapsed on maintenance, including 2 high-risk patients with complex karyotype and detectable MRD at enrollment. There were 3 deaths, all among relapsing patients. ELN-favorable patients (n = 11), with a high incidence of normal diploid karyotypes (11/11), NPM1 mutations (11/11) and IDH1/2 mutations (5/11), did particularly well, with no relapses observed so far within this group. Seven patients (26%) were MRD-positive at enrollment. Of these, 1 (14%) converted to MRD-negative on AZA-VEN alone and 3 (43%) converted only after aHSCT. The most common grade 3/4 toxicities were thrombocytopenia (15%), leukopenia (11%), and neutropenic fever (7%).
Conclusions: Maintenance therapy with AZA-VEN is a feasible and tolerable strategy in AML patients who have achieved CR following both high- and low-intensity induction regimens. Although longer follow-up is needed, these preliminary results demonstrate encouraging rates of RFS compared to historical expectations. This suggests low doses of AZA and VEN may be effective at controlling disease relapse while minimizing toxicity once CR is achieved.
Kantarjian: Ascentage: Research Funding; Jazz: Research Funding; NOVA Research: Honoraria; KAHR Medical Ltd: Honoraria; Aptitude Health: Honoraria; AbbVie: Honoraria, Research Funding; BMS: Research Funding; Pfizer: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Astellas Health: Honoraria; Astra Zeneca: Honoraria; Ipsen Pharmaceuticals: Honoraria; Daiichi-Sankyo: Research Funding; Immunogen: Research Funding; Amgen: Honoraria, Research Funding; Precision Biosciences: Honoraria; Taiho Pharmaceutical Canada: Honoraria. Borthakur: GSK: Consultancy; Takeda: Membership on an entity's Board of Directors or advisory committees; Astex: Research Funding; University of Texas MD Anderson Cancer Center: Current Employment; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Ryvu: Research Funding; ArgenX: Membership on an entity's Board of Directors or advisory committees; Protagonist: Consultancy. Yilmaz: Daiichi-Sankyo: Research Funding; Pfizer: Research Funding. Bose: Constellation Pharmaceuticals: Research Funding; NS Pharma: Research Funding; Pfizer: Research Funding; Sierra Oncology: Honoraria; CTI BioPharma: Honoraria, Research Funding; Novartis: Honoraria; Kartos Therapeutics: Honoraria, Research Funding; Celgene Corporation: Honoraria, Research Funding; Astellas: Research Funding; Promedior: Research Funding; Blueprint Medicines: Honoraria, Research Funding; Incyte Corporation: Honoraria, Research Funding; BMS: Honoraria, Research Funding. Jabbour: Amgen, AbbVie, Spectrum, BMS, Takeda, Pfizer, Adaptive, Genentech: Research Funding. Alvarado: Daiichi-Sankyo: Research Funding; Sun Pharma: Consultancy, Research Funding; FibroGen: Research Funding; BerGenBio: Research Funding; Jazz Pharmaceuticals: Research Funding; CytomX Therapeutics: Consultancy; MEI Pharma: Research Funding; Astex Pharmaceuticals: Research Funding. Pemmaraju: HemOnc Times/Oncology Times: Membership on an entity's Board of Directors or advisory committees; MustangBio: Consultancy, Other; CareDx, Inc.: Consultancy; Stemline Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; Cellectis S.A. ADR: Other, Research Funding; Plexxicon: Other, Research Funding; ASCO Leukemia Advisory Panel: Membership on an entity's Board of Directors or advisory committees; LFB Biotechnologies: Consultancy; DAVA Oncology: Consultancy; Novartis Pharmaceuticals: Consultancy, Other: Research Support, Research Funding; Sager Strong Foundation: Other; Springer Science + Business Media: Other; Abbvie Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Roche Diagnostics: Consultancy; Celgene Corporation: Consultancy; Samus: Other, Research Funding; ASH Communications Committee: Membership on an entity's Board of Directors or advisory committees; Aptitude Health: Consultancy; Affymetrix: Consultancy, Research Funding; Daiichi Sankyo, Inc.: Other, Research Funding; Incyte: Consultancy; Protagonist Therapeutics, Inc.: Consultancy; Clearview Healthcare Partners: Consultancy; Blueprint Medicines: Consultancy; Bristol-Myers Squibb Co.: Consultancy; ImmunoGen, Inc: Consultancy; Pacylex Pharmaceuticals: Consultancy. Takahashi: GSK: Consultancy; Novartis: Consultancy; Symbio Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Consultancy. Short: NGMBio: Consultancy; Jazz Pharmaceuticals: Consultancy; Novartis: Honoraria; AstraZeneca: Consultancy; Astellas: Research Funding; Takeda Oncology: Consultancy, Research Funding; Amgen: Consultancy, Honoraria. Issa: Novartis: Consultancy, Research Funding; Syndax Pharmaceuticals: Research Funding; Kura Oncology: Consultancy, Research Funding. Jain: ADC Therapeutics: Honoraria, Research Funding; Servier: Honoraria, Research Funding; Aprea Therapeutics: Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Cellectis: Honoraria, Research Funding; Janssen: Honoraria; Fate Therapeutics: Research Funding; Pfizer: Research Funding; TG Therapeutics: Honoraria; Pharmacyclics: Research Funding; Adaptive Biotechnologies: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Beigene: Honoraria; AstraZeneca: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Precision Biosciences: Honoraria, Research Funding; Incyte: Research Funding. Ferrajoli: Janssen: Other: Advisory Board ; AstraZeneca: Other: Advisory Board, Research Funding; BeiGene: Other: Advisory Board, Research Funding. Burger: TG Therapeutics: Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; AstraZeneca: Consultancy; Beigene: Research Funding, Speakers Bureau; Pharmacyclics LLC: Consultancy, Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; Gilead: Consultancy, Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; Novartis: Other: Travel/Accommodations/Expenses, Speakers Bureau; Janssen: Consultancy, Other: Travel/Accommodations/Expenses, Speakers Bureau. Daver: Bristol Myers Squibb: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Novartis: Consultancy; Abbvie: Consultancy, Research Funding; Sevier: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; FATE Therapeutics: Research Funding; Astellas: Consultancy, Research Funding; Trovagene: Consultancy, Research Funding; Trillium: Consultancy, Research Funding; Glycomimetics: Research Funding; Gilead Sciences, Inc.: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Hanmi: Research Funding; Amgen: Consultancy, Research Funding; Novimmune: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Other: Data Monitoring Committee member; Dava Oncology (Arog): Consultancy; Celgene: Consultancy; Syndax: Consultancy; Shattuck Labs: Consultancy; Agios: Consultancy; Kite Pharmaceuticals: Consultancy; SOBI: Consultancy; STAR Therapeutics: Consultancy; Karyopharm: Research Funding; Newave: Research Funding. DiNardo: Takeda: Honoraria; AbbVie: Consultancy, Research Funding; ImmuneOnc: Honoraria, Research Funding; Forma: Honoraria, Research Funding; Agios/Servier: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria; Foghorn: Honoraria, Research Funding; Celgene, a Bristol Myers Squibb company: Honoraria, Research Funding. Konopleva: Sanofi: Other: grant support, Research Funding; Forty Seven: Other: grant support, Research Funding; Calithera: Other: grant support, Research Funding; Rafael Pharmaceuticals: Other: grant support, Research Funding; Ascentage: Other: grant support, Research Funding; AstraZeneca: Other: grant support, Research Funding; F. Hoffmann-La Roche: Consultancy, Honoraria, Other: grant support; Agios: Other: grant support, Research Funding; AbbVie: Consultancy, Honoraria, Other: Grant Support, Research Funding; Stemline Therapeutics: Research Funding; Eli Lilly: Patents & Royalties: intellectual property rights, Research Funding; Ablynx: Other: grant support, Research Funding; Cellectis: Other: grant support; Genentech: Consultancy, Honoraria, Other: grant support, Research Funding; KisoJi: Research Funding; Novartis: Other: research funding pending, Patents & Royalties: intellectual property rights; Reata Pharmaceuticals: Current holder of stock options in a privately-held company, Patents & Royalties: intellectual property rights. Sasaki: Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees. Ravandi: AstraZeneca: Honoraria; AbbVie: Honoraria, Research Funding; Astex: Honoraria, Research Funding; Taiho: Honoraria, Research Funding; Agios: Honoraria, Research Funding; Jazz: Honoraria, Research Funding; Prelude: Research Funding; Novartis: Honoraria; Xencor: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Syros Pharmaceuticals: Consultancy, Honoraria, Research Funding. Kadia: Aglos: Consultancy; Pfizer: Consultancy, Other; Ascentage: Other; Cellonkos: Other; Genfleet: Other; Amgen: Other: Grant/research support; AstraZeneca: Other; BMS: Other: Grant/research support; Sanofi-Aventis: Consultancy; Genentech: Consultancy, Other: Grant/research support; Liberum: Consultancy; Jazz: Consultancy; Novartis: Consultancy; Astellas: Other; Dalichi Sankyo: Consultancy; Cure: Speakers Bureau; AbbVie: Consultancy, Other: Grant/research support; Pulmotech: Other.
Azacytidine SQ/IV and Venetoclax are not currently approved for maintenance in AML
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal